CXC Home | Search | Help | Image Use Policy | Latest Images | Privacy | Accessibility | Glossary | Q&A
Electromagnetic Radiation & Electromagnetic Spectrum
The word light usually makes one think of the colors of the rainbow or light from the Sun or a lamp. This light, however, is only one type of electromagnetic radiation. Electromagnetic radiation comes in a range of energies, known as the electromagnetic spectrum. The spectrum consists of radiation such as gamma rays, x-rays, ultraviolet, visible, infrared and radio.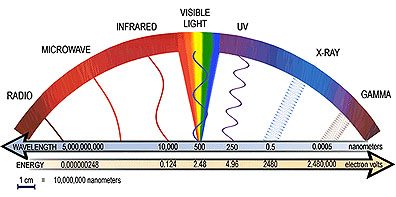
Electromagnetic radiation travels in waves, just like waves in an ocean. The energy of the radiation depends on the distance between the crests (the highest points) of the waves, or the wavelength. In general the smaller the wavelength, the higher the energy of the radiation. Gamma rays have wavelengths less than ten trillionths of a meter which is about the size of the nucleus of an atom. This means that gamma rays have very high-energy. Radio waves, on the other hand, have wavelengths that range from less than one centimeter to greater than 100 meters (this is bigger than the size of a football field)! The energy of radio waves is much lower than the energy of other types of electromagnetic radiation. The only type of light detectable by the human eye is visible light. It has wavelengths about the size of a bacteria cell, and its energies fall between those of radio waves and gamma rays. [More Info: Field Guide]
Return to E


